How To Calculate Sample Size For Hypothesis Testing
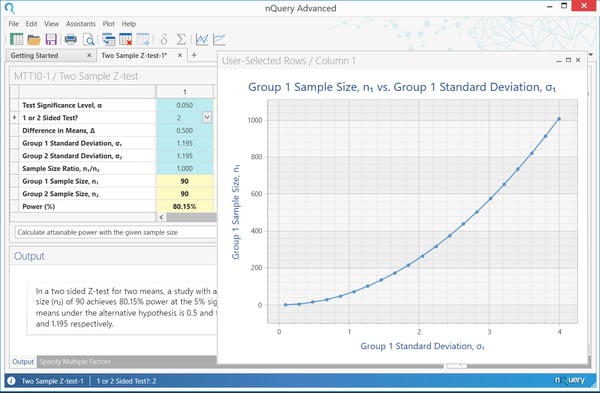
Once steps ane to 4 have been completed, and the appropriate sample size or relevant ability has been found, you can motility onto footstep 5 which is to explore the dubiousness in your sample size design . The unknown parameters and effect size that have been defined in steps 2 and iii are just that - estimates. It is not known what the true value of these parameters should be. If all these parameters were known, at that place would be no need to run the clinical trial! If the parameters are inaccurate, we take a chance the possibility of underpowering the study and non having a big enough sample size to find the consequence size or we may overpower and discipline besides many people to what may be an ineffective handling. Traditionally, this uncertainty would have been explored primarily using sensitivity assay. A sensitivity assay is a part of planning a clinical trial that is easily forgotten only is extremely of import for regulatory purposes and publication in peer-reviewed journals. Information technology involves analyzing what effect irresolute the assumptions from parts 2, 3 and 4 would have on the sample size or power in the particular sample size or ability adding. This is important as it helps in understanding the robustness of the sample size estimate and dispels the common overconfidence in that initial estimate. Some parameters have a large degree of uncertainty about them. For case, the intra-cluster correlation is frequently very uncertain when based on the literature or a airplane pilot study, and and then information technology's useful to look at a large range of values for that parameter to see what issue that has on the resulting sample size. Moreover, some analysis parameters will have a disproportionate upshot on the concluding sample size, and therefore seeing what effect even pocket-size changes in those parameters would have on the final sample size is very of import. When conducting a sensitivity assay, a choice has to be made over how many scenarios will be explored and what range of values should be used. The number of scenarios is usually based on the corporeality of uncertainty and sensitivity to changes and when these are larger, more than scenarios should be explored. The range of values is usually based on a combination of the evidence, the clinical relevance of different values and the distributional characteristics of the parameter. For case, it would exist common to base of operations the overall range on the range of values seen for a parameter seen beyond a wide range of studies or to base it on the hypothetical 95% conviction interval for the parameter based on previous data or a pilot study. For effect size, clinically relevant values volition tend to be an important consideration for which range of values to consider. Nonetheless, it is of import to note that there is no set rules for which scenarios should be considered for a sensitivity analysis and thus sufficient consideration and consultation should be used to define the breadth and depth of sensitivity suitable for the sample size determination in your study. A sensitivity assay for the example above is shown below. Here, the standard deviation in the group receiving the new handling is varied, to assess the outcome on the sample size required in that group. The sample size in the command group remains at 90, and we are always aiming for 90% ability. The plot shows that as the standard difference increases, the sample size required increases dramatically. If the standard deviation is underestimated, a larger sample size is required to achieve 80% ability, and thus the trial volition be nether powered. For σ= 1.5, i = 142, while for σ= 2.0, 1 = 253. This shows the importance of estimating the standard deviation as accurately as possible in the planning stages, every bit it has such a large impact on sample size and thus power. Though sensitivity analysis provides a nice overview of the outcome of varying the effect size or other analysis parameters, it does not present the full picture. It usually only involves assessing a minor number of potential alternative scenarios, with no set official rules for choosing scenarios and how to option between them. A method often suggested to combat this problem is Bayesian Assurance . Although this method is Bayesian past nature, information technology is used as a complement to frequentist sample size determination . Assurance, which is sometimes chosen "Bayesian power" is the unconditional probability of significance, given a prior or prior over some particular set of parameters in the calculation. These parameters are the aforementioned parameters detailed in steps 2 and three to a higher place. In applied terms, assurance is the expectation of the power over all potential values for the prior distribution for the effect size (or other parameter). Rather than expressing the effect size every bit a single value, information technology is expressed equally a hateful (the value the effect size is most likely to exist - usually the value used in the traditional power calculation) and a standard deviation (expressing your uncertainty about that value). If the power is then averaged out over this whole prior, the effect is the assurance. This is often framed as the "true probability of success", "Bayesian Power" or "unconditional power" of a trial. In a sensitivity analysis, a number of scenarios are chosen by the researcher, and assessed individually for ability of sample size. This gives a articulate indication of the merits of the private highlighted cases, but no information on other scenarios. With balls, the boilerplate power over all plausible values is determined by assigning a prior to one or more parameters. This provides a summary statistic for the consequence of parameter uncertainty, but less information on specific scenarios. Overall, assurance allows researchers to take a formal approach to accounting for parameter uncertainty in sample size determination and thus create an opportunity to open a dialog on this event during the sample size determination process. The definition of the prior distribution also allows an opportunity to formally engage with previous studies and skilful stance via approaches meta-assay or skillful elicitation frameworks such as theSheffield Elicitation Framework (SHELF) . O'Hagan et al. (2005) give an instance of an assurance calculation for assessing the effect of a new drug in reducing C-reactive protein (CRP) in patients with rheumatoid arthritis. " The outcome variable is a patient'southward reduction in CRP later four weeks relative to baseline, For the calculation of assurance, we suppose that the elicitation of prior information … gives the mean of 0.ii and variance of 0.0625. If nosotros presume a normal prior distribution, we tin can compute assurances with m = 0:2, v = 0.06 … With northward = 25, we discover assurance = 0.595 ." This calculation shows that a sample size of 25 per group is needed to achieve ability of 80%, for the given situation. The assurance calculation can then be demonstrated using the "Bayesian Assurance for Ii Group Test of Normal Means" tabular array. To view the list ofBayesian Sample Size Proceduresin nQuery, click hither. nQuery is the standard for fixed-term, Bayesian & Adaptive trials Pace 5. Explore Parameter Uncertainty
How can nosotros ameliorate upon or supplement the process of sample size determination?
What is Bayesian Balls?
How does Bayesian Assurance permit us explore incertitude?
What is an instance of an Assurance/Bayesian Power calculation for assessing the upshot of a new drug?
and the principal analysis will be a one-sided test of superiority at the ii.5%
significance level. The (two) population variance … is assumed to be … equal to
0.0625. … the test is required to have fourscore% power to detect a treatment upshot of 0.2,
leading to a proposed trial size ofnorthward one = n 2 = 25 patients … " 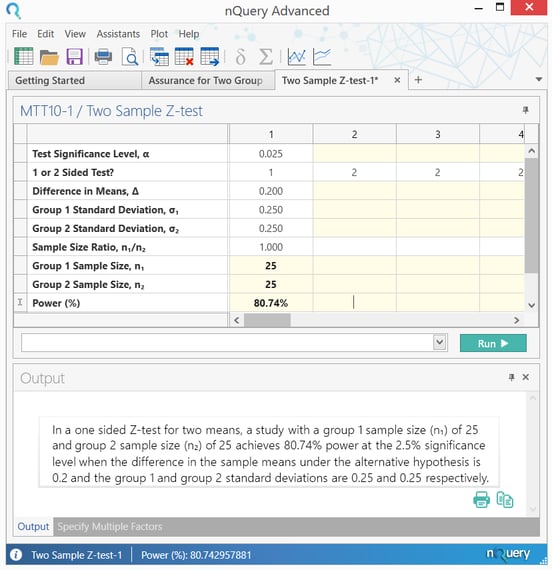
Start your 14 day costless trial of nQuery
How To Calculate Sample Size For Hypothesis Testing,
Source: https://www.statsols.com/how-to-use-a-sample-size-calculator
Posted by: hilliardpentor.blogspot.com


0 Response to "How To Calculate Sample Size For Hypothesis Testing"
Post a Comment